Geological and Technical notes.
Introduction
Henry Newton started his business in Gnosall in 1855. The earliest newspaper advertisement placed by him found so far is dated
1863. It gives an indication of variety of the building products that he was manufacturing at that time and is shown below:
The following account gives a “base level” explanation of how the minerals and rocks that are used as raw materials were mined,
transported and then processed in plaster and cement mills to manufacture those products.
However, the account begins with a consideration of the local geology around Chellaston, near Derby (where the Newton family
originated), and the Fauld area, near Tutbury, in East Staffordshire. These two areas are “famous” for their deposits of alabaster,
as well as gypsum and anhydrite, the raw materials needed to make plasters and some types of cement.
The manufacture of other types of cements that are derived from limestones such as Portland Cement are also covered.
Geological Setting
Some 180 - 190 million years or so ago, during the late Triassic period, Staffordshire was located at similar latitudes to the
Sahara Desert. The climate was hot and arid, and the landscape was one of low hills, with sedimentary basins, wide flood plains
and large shallow onshore ephemeral lakes, termed “Playa Lakes”.
The basins were fed by rivers arising in once mountainous areas, to the south in France. The rivers transported clays, muds and
silts (occasionally sands) as suspended sediments and deposited them into the basins. These sediments were lithified millions of
years ago and became the thick sequences of reddish-brown coloured mudstones and shales found all across the Midlands,
including Gnosall, where they are partly exposed in Cowley Cutting. The sequence is known as the Mercia Mudstone Group
(MMG).
In addition to the suspended sediment loads, rivers also carry minerals in solution, (dissolved out of the rocks over which they are
flowing). Where the rivers drained into playa lakes (as opposed to the sea) because of the hot climatic conditions these lakes
were susceptible to water loss by evaporation, and the consequent increase in concentration of dissolved minerals.
At the saturation point, the minerals start come out of solution as precipitates and then sink to the bottom of the lake. Sometimes
the playa lakes will dry up completely, creating “mineral flats” until river flows recover, and the lakes form again.
These river and climatic conditions prevailed over millions of years and the process was repeated many times. Not surprisingly
mineral deposits such as: Gypsum, Anhydrite and Alabaster formed in this way are collectively termed “Evaporites”.
Within Staffordshire the most important deposits of Gypsum, Anhydrite and Alabaster are found around Tutbury and near a
hamlet called Fauld in the Parish of Hanbury (there are others near Stowe by Chartley). Extensive deposits were also found in
Chellaston, where Henry Newton was born, some 5 miles SE of Derby City Centre.
The British Geological Survey now refer to all of the deposits (regardless of actual geographical location) as The Tutbury Gypsum
in their “Lexicon of Named Rock Units”.
The British Geological Survey Maps: Sheet 140 “Burton Upon Trent” covers Tutbury and Fauld areas and Sheet 141
“Loughborough” covers Chellaston. Both maps, together with vertical sections, cross-sections etc are available to view via the
BGS Website’s. Map Portal.
Evaporitic Minerals and Rocks
Gypsum
Gypsum is a naturally occurring, whitish, non-metallic mineral composed essentially of Calcium Sulphate. It also contains two
molecules of water incorporated within its molecular structure (known as the Water of Crystallisation). The chemical formula is:
CaSO
4.
2H
2
O. In nature it can found in both crystalline and in massive forms. Gypsum is very soft and is easily scratched. It is
designated as the Index Mineral Hardness 2 on Moh’s Scale of Hardness (where Talc = 1 and Diamond = 10).
Anhydrite
Anhydrite is a naturally occurring mineral very often found associated with gypsum. Like gypsum it is essentially Calcium
Sulphate, but, as its name suggests, does not have any water of crystallisation. Its chemical formula is CaSO
4
. It tends to be
greyish in colour but is harder than gypsum, registering 3 to 3.5 on the Moh’s Scale.
Alabaster
Alabaster is not a mineral per se, it is a rock composed a range of constituent minerals in varying amounts. The minerals include
Gypsum, Anhydrite, Calcite (Calcium Carbonate, CaCO
3
) and traces of other minerals. It has a characteristic mottled and/or
colour-banded appearance. It is a soft rock and can be carved, sculpted, turned on lathes and polished. It has been used for
centuries to make ornate architectural features and “objets d’art”.
Although gypsum, anhydrite and alabaster are classified as different substances, in evaporitic mineral deposits they are often
found blended together in complex intermingled irregular bodies rather than individual distinct beds of pure gypsum or alabaster.
Tutbury Gypsum
In 1906 Thomas Trafford Wynne, an experienced Mining Engineer (Henry Newton’s son in law) presented a paper to the
Institution of Mining Engineers titled “Gypsum in the Dove Valley”, where he described the nature of the Tutbury Gypsum
deposits.
Extracts as follows:
The gypsum is found cropping out on the hillside and has proved to be a continuous though irregular deposit, though how far
back into the hill has not yet been ascertained...
All classes of stone are mined: large alabaster blocks, either pure white or veined or coloured; and from the best white gypsum
from which the highest grades of plaster are made, through the various grades down to stone so mixed with marl as to be
valueless.
Anhydrite, or as it is called locally “hardstone”, is met with in varying quantities…There seems to be no dividing line or clear
cleavage between the gypsum and the anhydrite. The hardstone is often intimately mixed with the best gypsum stone, it has the
same appearance, and it is only by testing with the pick that the hardstone can be detected.
Mines and Mills
Chellaston
Henry Newton was born in Chellaston, and research has shown his parents and grandparents were landowners, and that several
generations of the wider Newton family were involved in the plaster and cement trade.
Alabaster and gypsum deposits were found very close to the surface at Chellaston making them easy to quarry, and later on
when quarrying became impractical the deposits were mined.
Alabaster from Chellaston was worked for centuries and in the 1600s there was a thriving export trade to Europe via Hull.
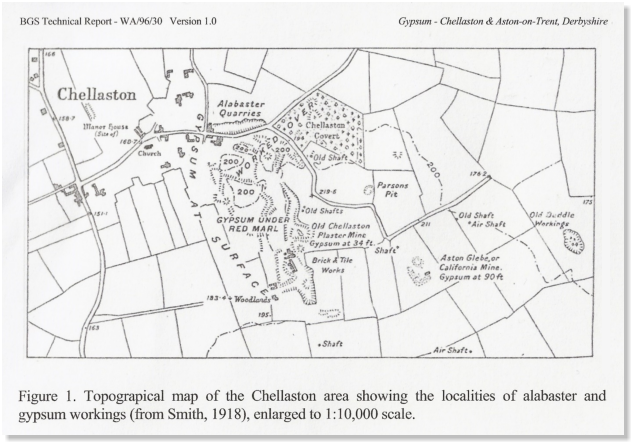
This Map is taken from a British Geological Survey Technical Report and gives an indication of the scale of the quarrying and
mining in the Chellaston by the 1800s:
Other authors note that gypsum was transported from Chellaston to be processed a few miles away in Burton on Trent, initially at
Union Mills but then some time later at Shobnall Mills, owned by the Newton family, and situated on a side arm and wharf of the
Trent and Mersey Canal, which opened in 1777.
Fauld
As discussed earlier the Tutbury Gypsum also occurs in East Staffordshire in the Parish of Hanbury near a hamlet named Fauld.
There were three mines in the Parish: one at Draycott in the Clay and two near Fauld.
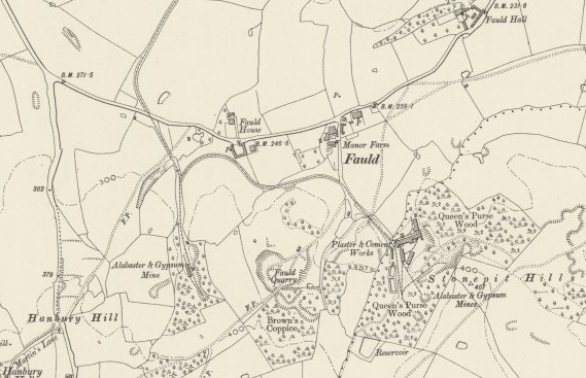
The 1901 OS 6-inch Map extract shows the mines at Fauld:
The mine on the east side of Fauld Hill belonged to Peter Ford and Son, and as can be seen, at that time it had its own plaster
and cement works.
The westerly mine belongs to JC Staton & Co. owned by the Staton / Newton family. Gypsum etc from there was for many years
transported to the Shobnall Works in Burton on Trent for processing into plaster, and from where it could be sent to customers via
the canals, and later on by the railway network.
Henry Newton Jnr (1853 – 1935), married Helen Agnes Wynne (Thomas Trafford Wynne’s sister) at St Lawrence’s Church in
1886, and shortly after moved from Gnosall to the Burton on Trent area to manage the family business at Shobnall and Fauld.
In 1888, Henry Jnr leased a strip of land and built a 3ft gauge mineral railway from the mine as shown on the map. The railway
terminated at the company’s exchange sidings at Scropton (off the map to the north). Bagnall’s of Stafford supplied the
locomotives.
Henry also built the branch line to the Ford’s mine and works, and transported plaster from Ford’s site to the sidings at Scropton.
The financial details of the agreement are unknown, but the arrangement lasted until 1944.
In 1901, Henry Jnr became solely responsible for the overall control of JC Staton & Co. He set about converting the disused
Tutbury Cotton Mill into a Plaster and Cement Works and constructed another 3 ft gauge railway from Tutbury Mill to Scropton.
He also brought in his brother-in-law Thomas Trafford Wynne (the mining engineer) to manage the mining operations at Fauld.
In his 1906 paper, Thomas T Wynne describes the methods of mining:
The roof of the deposit is composed of hard marl, it stands very well when dry, but in wet places a layer from 18 inches to 3 feet
soon sags, where a road is required to be kept open the marl must be either pulled down or timbered with oak props.
There is nothing special about the methods of getting out the gypsum stone for the mills. The system adopted is a kind of Pillar
and Stall. There is, however, not the regularity in size of the stalls and pillars that is usual in coal mines due to the variations in
the gypsum and in the roof conditions.
The great aim is to leave as little good stone as possible in the mine and leave the pillars, so far as is possible, where the inferior
stone is found. The rock is soft and easily bored with auger-drills by hand, there is need of mechanically operated drilling plant. …
Powder is used for blasting.
And
The method of obtaining blocks of alabaster is more complicated, as it is of the utmost importance that they shall not be in any
way shaken by blasting. Where good solid rock occurs, and it appears suitable for cutting into alabaster blocks, all blasting in the
immediate neighbourhood is stopped.
The rock is first “topped” – the roof is undercut just above the good stone and then blown down with lightly charged shots for
about 4 – 5 feet back and about 2 -3 feet in height to create space for the workmen.
A gutter about 10 inches wide is cut by the workmen along the back of the block, for such a length as may be considered
advisable, and others at each end, so that the block, generally 4 feet wide and between 4 and 20 feet in length, is separated from
the rest of the rock except at the bottom.
The thickness of the block is then determined, usually it will be half the thickness of the rock, so as to obtain 2 blocks of equal
size. Lines are marked along the block and auger-holes are bored through it. Steel feathers and wedges are inserted, and the
block is forced off its bed. It is then turned over, inspected and any inferior stone dressed off.
If too large, or if one end proves to be inferior or shaken, then blocks are sawn into such lengths as may be desirable, and it is
then ready to be loaded on to waggons and sent to the artist or manufacturer. Every block does not always turn out to be good
alabaster, and a high percentage prove, after going to considerable expense, to be valueless.
This photograph of blocks of alabaster from Fauld loaded on railway wagons has been “borrowed” from the Tutbury: Local History
and Information website.
This photograph of blocks of alabaster from Fauld loaded on railway wagons has been “borrowed” from the Tutbury: Local History
and Information website, which contains an account of the mines, the manufacture of plaster at Tutbury Mill and other interesting
historic photographs.
A gypsum mine at Fauld is still working today under the control of British Gypsum (part of St Gobain) and it supplies “gypsum /
anhydrite rock” to many of the UK’s mass production cement plants, that use it as an additive in the raw material mix (rawmix).
From a historical perspective the Fauld area is probably best remembered as the location where in 1944 there was a huge
accidental explosion of munitions that were being stored in the mine workings. The blast caused the formation of the “Fauld
Crater” which is over 300m across and 30m deep and now forms a significant landscape feature.
Further information and photographs regarding the Fauld incident can be found on the Tutbury Local History and Information
website, which contains an account of the mines, the manufacture of plaster at Tutbury Mill and other interesting historic
photographs..
Processing and Products
Plaster and cements in one form or another have been used in the construction of buildings and other structures, for example
bridges, since they started to be built from stone and bricks.
Plaster is the general term for the building material that is applied to the internal walls, ceilings and sometimes floors of buildings.
It improves insulation and makes the interior walls easier to decorate and ornament.
The word “cement” is derived from “caementum” which is the Latin word for masonry; and in historical times was used to describe
the material used to bond the individual stone blocks or bricks together. Nowadays, it is more usual to call this “mortar”.
What all plasters and cements have in common is that the raw material is either a naturally occurring mineral or rock, or a
combination of minerals and rocks. These will have been mined or quarried, crushed into a manageable size and put into a
vessel above a hearth and heated, or loaded into a kiln and fired, sometimes called burning.
“Calcining” is the technical term for this heating or firing process, and it brings about chemical changes in the raw materials. The
resultant fired product is then milled and ground into a fine powder to make either the plaster or the cement. Once made they
must be kept and stored in dry conditions.
When required for use, water is added to the dry plasters and cements. Chemical reactions are initiated causing the wet mixtures
to “go off” or set hard. The amount of water in any mix can be varied according to the desired application. Stiff pastes would be
required for plastering walls, whereas wetter mixes that flow would be needed for pouring into moulds to make castings.
Throughout the Industrial Revolution and beyond, scientists and engineers sought to make new and better cements by
experimentation. They tried using different minerals and rocks, singly or in combination, they varied the duration, and the
temperatures of the heating or firing process. This resulted in a wide range of “patented or specialist products” coming to the
market as is shown in the advertisements placed by Henry Newton.
These named cements are either made from gypsum type rock, or from limestones that have had other rocks and minerals such
as clays mixed in with them before firing. For example: “Keene’s Cement” is made from Gypsum and is technically a type of
plaster, whereas “Roman Cement” is made from argillaceous muddy limestone.
In the early days, when production control was somewhat primitive, the quality of the finished cements and plasters may well
have been “a bit hit and miss”. In more modern times production is much more scientifically managed, but the basic process is
essentially the same, and it has not changed since the 1750s.
Gypsum-based Plasters and Cements
As previously outlined, the production process involves crushing and milling the Gypsum into a powder and then heating it to
“drive off” some or all of the water of crystallisation. The temperature and the duration of the heating determines how much water
of crystallisation is removed and determines the nature of the resultant plaster.
For
example:
heating
raw
gypsum
to
about
150
degrees
for
about
an
hour
produces
Plaster
of
Paris:
(Calcium
Sulphate
Hemi-
Hydrate,
CaSO
4
·1/2H
2
O).
Plaster
of
Paris
was
used
extensively
in
the
ceramic
industry
to
make
moulds
for
slip
casting
and
when
the Potteries were in their heyday it was in great demand. It is also used for medical and dental applications.
Historically
the
production
of
architectural
and
building
plasters
such
as
Keene’s
cement
required
all
of
the
water
of
crystallisation
to be driven off by heating the raw gypsum for longer and at higher temperatures.
Nowadays
the
majority
of
building
plasters
are
made
from
Plaster
of
Paris.
Chemical
retardants
are
added
into
finished
product
to
extend the setting time, thus enabling plasterers to keep batches of wet plaster active for longer.
Temperature and duration are not the only factors: the composition of the raw gypsum is also critically important. Thomas Trafford
Wynne’s account of the Fauld mine describes the contrast between “best gypsum” and the lower grades that have varying
amounts of gypsum and anhydrite (Hard Stone), intimately mixed together.
In the 1850s and ’60s Henry Newton’s works would have used large steel pans (sometimes called kettles), perhaps 10 to 12 feet
in diameter, to heat batches of raw gypsum. The pans would have sat on open hearths, which would be fed with coal. The hearths
would have been equipped with flues to create an updraught and improve combustion, and also help to remove smoke and
fumes.
Whilst being heated, the raw gypsum would have been stirred to ensure that the heating was even across the whole extent of the
pan and the required temperature was achieved consistently. In the early days the stirring would have been done by hand by
workers called “plaster boilers”. In later years, some plaster mills used pans equipped with mechanical shakers; it is not known if
Henry Newton ever installed any these.
It is thought that Henry Newton had perhaps 4 or 5 hearths and pans, and that to avoid cross contamination, they were each
assigned to be used only for a particular grade of raw gypsum. It has been noted that “Ceiling and Wall Plasters” were made
from finer grades than that used to make “Floor Plaster”. The poorest quality would have been sold as fertiliser, and it may not
have been heated, just crushed and milled into powder.
The “best gypsum” would have been used to make Plaster of Paris for medical and dental applications. The gypsum was heated
in closed box ovens as these were more controllable than hearths and also it reduced the chance of contamination of the finished
product by coal dust and other airborne impurities that may have been present in the works.
Keene’s Cement
Keene’s Cement was developed and patented by Richard Wynn Keene and a John Danforth Greenwood in 1838. (Danforth
emigrated to New Zealand in 1843).
They made the cement by steeping powdered Gypsum in a solution made up of a mixture of Aluminium Sulphate and Potassium
Sulphate and calcining it at about 170 degrees. The resultant compound was then ground down to a fine powder to produce the
finished cement.
In use, it is mixed with water, applied to the task and then allowed to dry in the normal way. The formulation produces a very hard
plaster which can be polished and easily cleaned. Pigments could also be added to produce coloured plasters.
Due to its hardness when dry, it was often used in moulds to produce ornate plaster castings such as skirtings, dado rails, ceiling
roses and other decorative features.
Martin’s Cement & Parian Cement
Martin’s Cement and Parian Cement are very similar products, both developed in the 1800s. They were made by adding Borax
(Na
2
B
4
O
7
10H
2
O) to the raw gypsum before heating in the usual way.
In use, they make hard glossy white cements, similar to Keene’s cement, and are used for the same sorts of architectural plaster
products.
-----------------------------------
Henry Newton’s 1884 block advertisement (shown in the main account) is repeated here for the benefit of readers. Note the
difference in the variety of the products compared to his 1863 advertisement shown at the start of this account.
Limestones
Mineralogically all limestones must contain a high percentage of Calcium Carbonate: CaCO
3
(otherwise the rock wouldn’t be
classified as a limestone). In Britain they are found in most of the geological time periods (Silurian, Carboniferous, Jurassic etc)
and are often a reflection of the latitude of Britain at that time in geological history.
Limestones vary considerably in mineralogical composition: some “Pure Limestones” such as those found near Buxton in the
White Peak contain upwards of 90% of CaCO3 where as others, for example: the Blue Lias, which outcrops around Rugby, would
be classified as “Argillaceous (muddy) Limestones” where thin mudstones occur interbedded with the limestones, giving a
mineralogical composition of about 70% of CaCO
3
and 30% clay minerals; and “Dolomitic Limestones” (Dolostones) can have
broadly equal amounts of Calcium Carbonate and Dolomite: Magnesium Carbonate (MgCO
3
) in their constituent grains.
The colour, texture, properties and mineralogical composition of any limestone depends on the origin and nature of the
sediments, and the prevailing physical, chemical and environmental conditions at the time that the sediments were being
deposited.
Lime
“Lime”-based products have been manufactured from Limestones by calcining for centuries, and the remains of lime kilns can be
found wherever there are outcrops of limestone. The firing process causes a chemical change: carbon dioxide CO
2
is released or
driven off, leaving “Quicklime” (Calcium Oxide: CaO) as the resultant product.
Although quicklime is used for some applications, it is chemically unstable and will absorb moisture from the atmosphere if left in
open containers, as well as moisture from the skin causing chemical burns. It is a dangerous product which needs to be handled
and stored with care and respect.
Mixing water and quicklime together cause an exothermic chemical reaction: large amounts of heat are generated by the reaction
which also converts the quicklime, Calcium Oxide into Calcium Hydroxide: Ca (OH)
2.
Consequently, water was often added to the quick lime (slaking) at the lime works to manage this chemical reaction, thus making
a much less dangerous and more handleable and transportable product.
Slaked Lime was used dry as “agricultural lime” to reduce soil acidity. It was also used to make Lime Mortars and Cements by
mixing it with sand and water. Lime mortar is comparatively weak when compared with later Roman and Portland Cements.
It was also mixed with water to make Lime Washes. Timber-framed buildings with walls made of “wattle” – thin laths of wood
woven together – and “daub”, a mud / clay paste, commonly had lime wash applied to the walls which helped with
weatherproofing, and improved the appearance.
Roman Cement
James Parker lived in Northfleet in Kent and had been calcining (burning) local limestones and marls for a number of years,
seeking to develop new and better cements. His first patent was granted in 1791 for his “Method of Burning Bricks, Tiles and
Chalk”.
In 1796 Parker was granted another patent for what he called “Roman Cement”. The cement was not produced by using
techniques first developed by the Romans: the finished product was pinkish in colour, similar to the stone used for buildings in
and around Rome and Parker just used the name.
Parker made Roman Cement by calcining broken “Septarian Nodules” in a bottle kiln. Septarian Nodules are hard calcareous
mudstone concretions that occur within clay formations, such as the London Clay. Being hard they tend to weather out from the
softer clay deposits and are commonly found as large pebbles and cobbles on beaches and in estuarine gravel deposits around
the coastline.
Contemporary reports state that the kiln was operated continuously, with equal volumes of nodules and coal being fed in at the
top and the calcined material being drawn off at the bottom. The kiln operated at about 1000 degrees and the residence time was
about 2 to 3 days.
Parker had a windmill at the site, which he used to grind the cooled calcined nodules into a fine powder to make the finished
cement. The cement needed to be packed into barrels quickly as it was moisture sensitive.
The patent lapsed in 1810 and many other cement manufacturers started to produce their own “Roman Cements”, as by then the
science behind cement processing had moved forward and it was understood that roman type cements could be produced from
muddy limestones or lime rich clays with similar mineralogical composition to Septarian Nodules.
When in use and combined with water, Roman Cement made a strong, hard, durable and weather resistant cement binder that
set within 5 to 15 minutes. It could be mixed with sand to make mortars for masonry and brickwork. Also, due to its weather
resistance and rapid setting time, it could be used on the exterior of buildings as a render (stucco) and was thought of as the
exterior equivalent of Gypsum Plaster.
Roman Cement was also used to make blocks of “artificial stone” which could be carved and sculpted, or it could be used to run-
mould and cast ornate architectural features. It was used extensively in the 1820s restoration of the exterior of Lichfield
Cathedral: for example, the seven statues above the south door are carved out of blocks of Roman Cement.
Portland Cement
Modern Portland Cement is defined as a calcareous cement that contains the chemical compound Alite (Tricalcium Silicate). It is
made by carefully proportioning limestone with clay to achieve a “cement rawmix” that contains about 75% to 80% of Calcium
Carbonate. The rawmix is then burned at a high temperature, well above 1250 degrees (otherwise Alite will not form).
The high temperature causes the rawmix to partially melt, and the resultant product to fuse together to form a clinker. This clinker
is then pulverised and ground down to produce the finished cement powder. When in use and the cement is mixed with water, it
is Alite that gives the cement its strength.
Joseph Aspdin, a bricklayer from Leeds, patented a product in 1824 (British Patent 5022: An improvement in the modes of
preparing artificial stone) which he called “Portland Cement” because the colour of the set mortar it made was pale grey similar to
“Portland Stone”.
Joseph Aspdin’s method used a “double burning” process. He calcined lumps of limestone to produce quick lime, which he then
slaked. Then he added clay and water to the slaked lime, thoroughly mixing them together to make a slurry. The slurry was dried
and then burned in a kiln for a second time and the fired residue was then ground down to make the cement.
Joseph Aspdin, and his two sons William and James, established their cement works in Wakefield. However, there is a consensus
amongst “informed technical writers” that Joseph Aspdin’s cement was not a true Portland Cement, in the sense that we
understand today, as it was not made from a clinkered Limestone and Clay mix.
Roman Cement plants had always produced small quantities of clinker due to “over-burning” and it was normally discarded. It is
thought it was Joseph’s son, William Aspdin, who through experiment discovered the significance of the clinker and actually
developed Portland Cement, as we now know it today.
William Aspdin moved to London in 1841, setting up a plant producing and selling what he called Portland Cement in 1842. It
appears that William Aspdin was somewhat of a “character”. William went to great lengths to keep his manufacturing process and
firing temperatures secret from potential competitors by producing false documents and misleading technical reports.
William also claimed that his product was made in the same way as his father’s, and he kept the Portland Cement name, in order
to gain the rights conferred by his father’s 1824 Patent. He also made outlandish claims about its usage and beneficial
properties: however independent testing did show that his Portland Cement was considerably stronger than the Roman Cements
available at that time.
Despite William’s efforts to keep the process a secret, other cement manufacturers were experimenting and learning how to
produce Portland Cement, and by the later 1800s it had become the market leader.
It became clear as the “new” cements particularly the Portland type Cements were being developed for large scale production,
that traditional open kilns used for lime burning were unsuitable, as the high temperatures needed to fire successfully could not
be achieved. Bottle Kilns had been used in the ceramics industry for high temperature firing since the 1700’s and so cement
manufacturers copied the technology and adapted it to suit their needs.
Bottle kilns for cement production first came into use in the 1840’s and were becoming industry standard practice by the time
Henry Newton was building the cement works in Gnosall. The kilns were essentially traditional eggcup shaped open lime kilns
with grate bars at the base, and lined with firebricks, but with the addition of bottle shaped brick tops, to improve draught and
combustion.
The kilns were built with an opening near to the top to facilitate loading and one near the base to enable recovery of the fired
product. Prior to firing, the openings would be bricked up to seal them, and then broken open at the end of the firing cycle.
To prepare the kiln charge the limestone had to be crushed and milled. It was then put into tank to which water and the right
amount of clay was added, and the whole mixed thoroughly to make a slurry. Excess water was drained off and when the slurry
became handleable it was moved to “drying floors” in the mill where it would be left until it was ready for the kiln.
During the loading, dried “slurry cake” and coal were placed the kiln in alternating layers. The firing cycle took around a week or
so: loading and unloading was done in a day (12-hour shifts) firing took around 2 days and 2 days for cooling down before
unloading, making 6 to 7 days in all. Sundays were not normally working days, and so firing cycles were scheduled to include
them as a day for cooling.
There a number of were early experiments to try to shorten the firing cycles, thus potentially improving output, but they were not
very successful. It was easier to fulfil a growing market demand just building another kiln.
The photograph of Henry Newton’s works shows three bottle kilns, all of which appear to be of different sizes, and it may be that
Henry started with one and built the other two at different times, as his business grew.
The above account has been put together from information in Dylan Moore’s very detailed and informative internet site: Cement
Kilns and is recommended to readers who may be interested in the history and development of the cement industry.



With grateful thanks to Mike Barton for this very detailed technical document and to Jeremy Manners
author of ‘History of Staton and Co’ web page for permission to use the content.